What is Periostin doing in the extracellular matrix?
Periostin interacts with extracellular structural molecules, such as Collagen Type I and cell membrane proteins, such as αvβ3 and αvβ5 integrins. These matricellular interactions have been reported to influence cell behavior as well as collagen fibrillogenesis6. The collagen fiber diameter is reduced in the absence of Periostin, resulting in a decrease modulus of elasticity in the KO. In addition, Periostin interaction with αvβ5 activates cell survival signaling pathways. Our group demonstrated that Periostin closely related with the PDL fiber bundles from the root surface cementum to the alveolar bone engaging the tooth supporting apparatus. Thus, in the context of Periostin deletion, the periodontium rapidly deteriorates over time and is unable to sustain the physiologic mechanical stimulus. Collectively this evidence suggests a critical role of the matricellular adaptor protein, Periostin, serving as a potential modulator of important tissue mechanical properties. Significant molecular changes have been reported to occur during periodontal disease progression. Altered levels of Transforming Growth Factorβ (TGF-β) have been implicated in periodontal disease progression42-45. TGF-β-1 reduced levels in advanced periodontal disease have been associated with altered Matrix Metalloproteinases (MMP’s) and Interleukin-1β (IL1-β) activity42,44. TGF-β-1 levels increase under mechanical strain and regulate the expression of Periostin26,41,46. These molecules are expressed at different stages of wound healing and some patterns are more transient than others. We have reported novel findings in regards to its relevance in PDL function40,41. Our data suggest that TGF-β may influence the integrity of the periodontium through the regulation of Periostin expression and function. The importance of Periostin in other specialized tissues, such as the heart, clearly highlights its importance in repair, regeneration and recovery after myocardial infarction. Periostin is normally secreted by PDL fibroblasts in response to injury, and it interacts with integrin receptors on target cells to modulate cellular and matrix remodeling. Recently, with the advances in technology and the availability of animal models, a more mechanistic model of disease pathogenesis appears feasible. From conventional deletion of periodontal “specific” genes we have identified key factors that appear to determine functional and structural stability of the supporting tissues. Mice lacking the transmembrane protein αvβ 6 integrin, a molecule constitutively expressed in the healthy junctional epithelium, lose rapid attachment and a significant apical migration of the sulcular epithelium favors the formation of periodontal pockets47. These changes are associated with altered signaling of TGFβ-1, a molecule involved in multiple regulatory functions including tissue repair and immune system. TGFβ-1 is secreted from the cell as a latent precursor complex. The secreted latent TGFβ-1 is bound to the extracellular matrix via a latent TGFβ-1 binding protein (LTBP1)48. Binding of αvβ6 integrin to the atent associated propeptide has been implicated in the activation and release of the latent TGFβ-149,50 which in turns favors extracellular matrix (ECM) formation and maturation by the regulation of MMP-8 and MMP-1342,51. The examination of tissues from advanced periodontal disease patients shows consistent changes that are characterized on one hand by decreased TGFβ-1 and αvβ6 integrin, and on the other hand by increased levels of tissue collagenases, gelatinases and proinflammatory cytokines such as IL1-β44,47,52,53. The junctional epithelium during periodontal disease is the first critical tissue barrier breached by oral bacteria. It then triggers a robust, leukocyte-mediated immunological defense reaction within the gingival tissues. This inflammatory reaction may be contained at this level or, as in the case of susceptible individuals, it may progress to compromise the PDL integrity and the alveolar bone.
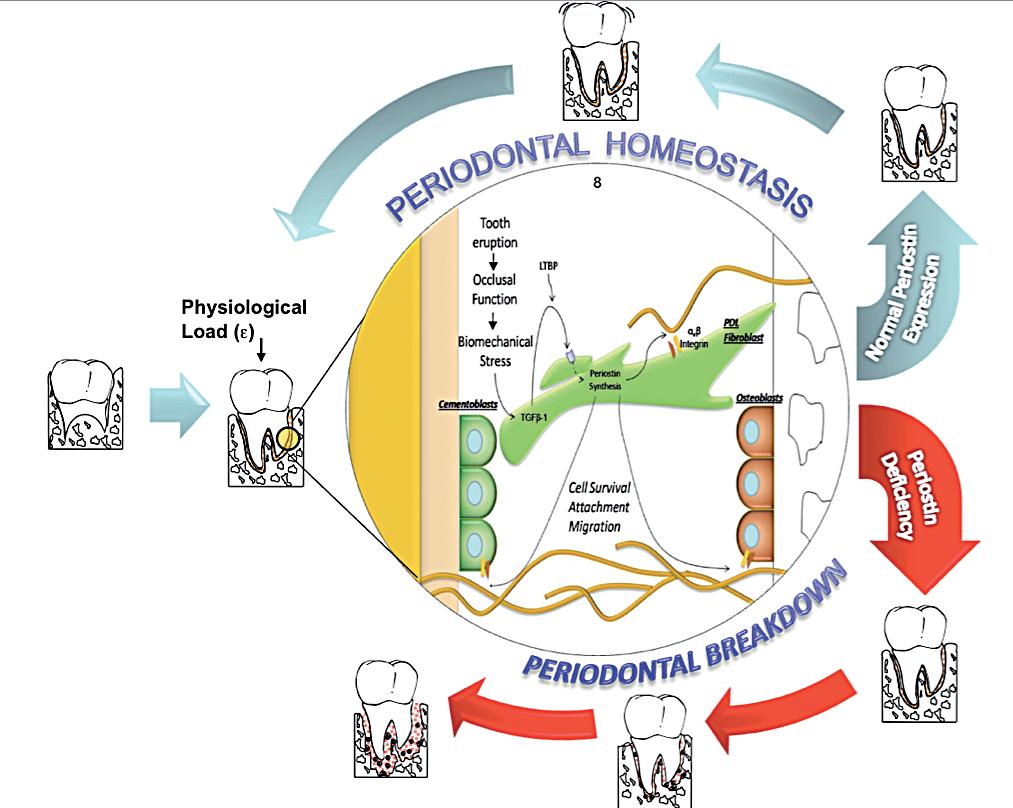
Discussion
Periodontal diseases affect a large proportion of the world’s population. The identification of genetic susceptibility variants and their role on disease onset and progression has been the focus of a number of studies. Based on the evidence reviewed, Periostin represents a novel biologic agent with significant diagnostic and therapeutic potential that could ultimately enhance patient care. The available preclinical and mechanistic studies currently available in the periodontal biology field, have provided provide novel insights into the regulation of PDL function during periodontal tissue health and disease. Understanding the role of Periostin in the PDL, helps capture the dynamic nature of, the matricellular biochemical events involved in the transition between periodontal health and disease (Figure 4). This information, further contributes to the ongoing effort of describing the basic elements involved in a new model of periodontal disease pathogenesis. Such a model incorporates gene, protein, and metabolite data into dynamic biologic networks that include disease initiating, susceptibility, and resolving mechanisms. Ultimately, these data will aid in our understanding of periodontal biology relevant to cell-matrix dynamics and homeostasis. Therefore, unraveling novel pathways that determine periodontal disease susceptibility that could be targeted in the treatment of inflammatory periodontal diseases. Physiologically relevant differences in Periostin levels are yet to be determined in humans. From our animal model experiments, we reported a clear periodontal phenotype developing in the mice completely lacking Periostin (KO) while minor or no changes occurred with a 30-50% decrease in Periostin (Het). However, these observations do not reflect a chronic microbial challenge condition such as the one find in human periodontal disease. The model proposed partially addresses this issue. However, the inability to induce the formation of a complex biofilm as in human periodontitis is a clear limitation. Currently, periodontal conditions are diagnosed based on the clinical presentation of the disease. The current classifications guides us to identify “different” forms of the disease that manifest themselves with a common clinical presentation and cluster them within groups (i.e., chronic, aggressive, necrotizing, etc). Therefore, it is our responsibility to acknowledge the complexity and heterogeneity of this group of conditions. The limitation of the lack of an etiology-based classification compromises the accuracy of a specific diagnosis and, therefore, the identification of the most appropriate therapy. Further studies should be geared to identify underlining conditions, such as Periostin expression, that may be associated with an increase rate and extent of periodontal breakdown.
Acknowledgements
The Authors appreciate Chris Jung for assisting with the preparation of the figures. This work was supported by the National Institute of Health/National Institute of Dental and Craniofacial Research grants NIH/NIDCR K23DE019872 (HFR).
Correspondence
Hector F. Rios
Department of Periodontics and Oral Medicine
University of Michigan, School of Dentistry 1011 N. University, Ann Arbor, MI 48109-1078
Tel. 734-763-3383 – Fax: 734-936-0374 – hrios@umich.edu





[…] >English Version> […]